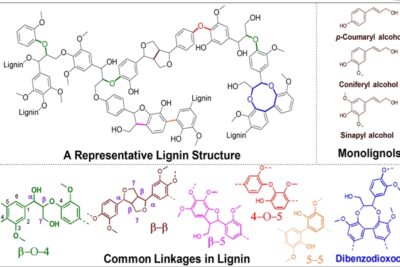
This working group focuses on the research and development of technologies for fractionation of raw materials from Estonia into pure polymers and analytical chemistry related to plant biomass. Plant biomass is heterogeneous and consists of three main polymers - cellulose, hemicellulose and lignin. If cellulose is about 40-50% of dry mass in wood, lignin is about half. Lignin is a branched polyphenolic polymer, the structure and composition of which vary considerably from species to species. In the case of lignin, we can talk about three basic units (G, S and H), which are joined to each other as the plant cell wall grows and thickens, resulting in the formation of this branched polymer. While in conifers lignin mainly consists of G units, in hardwoods there are almost equal amounts of G and S units.
In herbaceous plants, however, there are also H units, which allow lignin to branch even more, making this polymer perhaps the most complex in terms of its structure. The high content of benzene nuclei in lignin is one of the reasons why lignin is seen as a green and renewable alternative to petroleum-based chemistry. As a result of the green revolution, this topic is therefore very important. In order to convert any raw material into chemicals with added value as efficiently as possible, it is necessary to understand the chemical composition of this material. The working group for fractionation and analytical chemistry of wood polymers focuses largely on the topic of analytical chemistry, so that, based on the raw material, it is possible to easily determine, for example, the amount of different sugars in the biomass, as well as to determine what type of lignin it is and how many chemically functionalizable groups are in the given material, which could be used in the development of novel functional materials. in synthesis.